A primer on the origin, extraction, and emissions of everyone’s favorite fossil fuels
Three major forms of fossil energy together provide almost all the world’s energy: coal, oil (and the derived petroleum fuels), and natural gas. Moreover, humanity’s insatiable appetite for these fuels is the major driver of climate change. Therefore, it is essential to understand the fundamentals of these fuels: Where do they come from, what are their properties, and how do we extract them. Furthermore, it is generally understood that natural gas is the “cleanest” of the fossil fuels, while coal is the dirtiest. Again, we must ask why, and provide at least a basic answer. The purpose of this article is to answer (at least broadly) these questions, and to make the reader familiar with some key terms and concepts. In brief, the takeaways are:
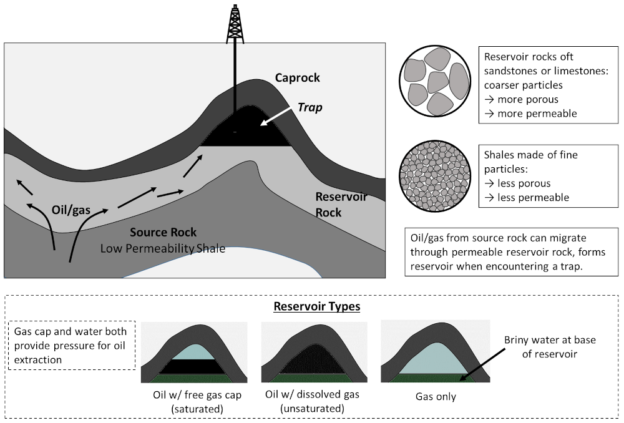
- All fossil fuels derive from organic matter buried at great depths and processed under pressure.
- Oil and gas are produced by the same basic geologic processes, and are often present in the same wells/fields. Conventional oil/gas is formed when these hydrocarbons seep from low permeability source shales to collect in “traps,” where they can be pumped by conventional methods.
- Unconventional oil/gas extraction via “fracking” involves drilling into and breaking up source shales directly.
- Coal in the US is most commonly mined via surface mines, especially mountain top removal with valley fill, where whole mountains are blasted away to access coal seams.
- Fossil fuels are mainly a mix of molecules with carbon (C) and hydrogen (H) bonds. Burning oxidizes these molecules to water and carbon dioxide, releasing heat along the way. The more C-H bonds, vs. C-C bonds, the more energy is released per atom of C, and thus the less CO₂ per heat energy released.
- Natural gas, as CH₄, has the most C-H bonds of any fossil fuel (it is the most “reduced”), and burns the cleanest. Coal is the least reduced fossil fuel, and burns dirtiest.